Abstract
Halogenated solvents (e.g. chlorobenzene and chloroform) are typically used for hole-transporting materials (HTMs) in perovskite solar cells (PSCs); however, their use should be avoided as they are known to be hazardous to the environment. Herein, we synthesized a nonhalogenated-solvent-soluble, dopant-free HTM, SF62. When depositing HTMs for PSCs, SF62 could be dissolved with a nonhalogenated and green solvent, ethyl acetate. It is one of the most common organic solvents and is known to have a low environmental impact. Non-doped-SF62-based PSCs exhibited higher power-conversion efficiency (18.6%) than doped 2,2',7,7'-tetrakis(N,N-di-p-methoxyphenylamino)-9,9'-spirobifluorene (Spiro-OMeTAD)-based ones (18.3%), with enhanced stability.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Metal halide perovskite solar cells (PSCs) have attracted considerable attention because of their scope for power-conversion efficiency (PCE) improvement and cost-effective solution processing. 1–3) The key components of a typical PSC include a compact TiO2 (c-TiO2) layer, mesoporous TiO2 (mp-TiO2) layer, hybrid organic–inorganic perovskite photo-absorber, hole-transporting material (HTM), and counter electrode (e.g. Au). The HTM is among the most important components of a PSC because it plays a crucial role in achieving high performance by allowing for charge recombination reduction. 4–8)
At present, 2,2',7,7'-tetrakis(N,N-di-p-methoxyphenylamino)-9,9'-spirobifluorene (Spiro-OMeTAD), a wide-band-gap material, is the most frequently used HTM, and devices based on Spiro-OMeTAD exhibit excellent performance. 9–11) However, pristine Spiro-OMeTAD films exhibit low conductivity and mobility. To overcome this limitation, dopants such as lithium bis(trifluoromethylsulfonyl)imide [Li(TFSI)], 4-tert-butyl-pyridine (TBP), and FK209 have been used for developing PSCs with high PCE. 12,13) Despite the enhanced hole transport properties, the doping strategy causes several problems. For example, commonly used dopants, such as Li(TFSI) and TBP, have a hygroscopic or volatile nature, which leads to accelerated degradation of PSCs. 14) These instability problems must be overcome for the commercialization of PSCs. 4,15)
Recently, our group reported a dopant-free cyano-substituted spiro-type HTM (SF48, Fig. 1) for PSCs. 16) The PSC with non-doped SF48 demonstrated higher thermal stability than that with doped Spiro-OMeTAD . Similarly, a series of dopant-free HTMs have been synthesized and investigated. 17–20) PSCs with such HTMs typically exhibit high PCE. However, these HTMs are processed with chlorinated solvents such as chlorobenzene (CB), dichlorobenzene, and chloroform (CHCl3). To achieve an ideal dopant-free HTM, an environment-friendly strategy for hole-transporting layer (HTL) preparation is preferred, similar to green-solvent processing. 21) Lee et al. reported a donor–acceptor copolymer-type HTM processed with a green solvent, 2-methylanisole. 22) Lu et al. reported a small-molecule HTM, which could be dissolved in nonhalogenated solvent tetrahydrofuran (THF). 23) However, a dopant-free HTM has not been widely fabricated using a green solvent. Moreover, there are very few solvents for HTMs in PSCs that do not damage the perovskite layer.
Fig. 1. Chemical structures of Spiro-OMeTAD, SF48, and SF62.
Download figure:
Standard image High-resolution imageNow, we developed a dopant-free green-solvent-processable HTM, SF62 (Fig. 1). We replaced the N,N-dimethylamino groups of SF48 with N,N-diethylamino groups to improve the solubility in green solvent such as ethyl acetate (AcOEt). AcOEt is one of the most common organic solvents and is known to have a low environmental impact. 24) Although both Spiro-OMeTAD and SF48 possess low solubility in AcOEt, SF62 is soluble. However, the perovskite layer composed of MAPbI3 (MA = CH3NH3) is insoluble in AcOEt; therefore, SF62 is a good candidate solvent for HTMs. We fabricated PSCs using non-doped SF62 and evaluated their performance. The stabilities of the cells were also examined.
The chemical structures of SF48 and SF62, including that of Spiro-OMeTAD, are presented in Fig. 1. SF62 was synthesized using a slightly modified version of our reported method. 16) The synthetic routes of SF62 are shown in Scheme S1, and its molecular characterizations are presented in Figs. S1 and S2. The optical and electrochemical properties of the HTMs are summarized in Table SI. The UV–vis absorption spectra of SF62 are shown in Fig. 2(a), along with those of the Spiro-OMeTAD and SF48. All HTMs absorb strongly in the 350–450 nm region, which is assigned to the π–π* transition. 25) The maximum absorption peak of SF62 is blue-shifted in relation to that of Spiro-OMeTAD. This phenomenon is attributed to the inductive electron-withdrawing effect induced by the cyano groups on the aromatic rings of the conjugated backbone. 26,27) Moreover, the absorption edge of SF62 extends into the long-wavelength region because the π-conjugated system is extended owing to the N,N-dimethylamino groups. The characteristics of SF62 are similar to those of SF48. The optical bandgaps (Eg) of SF62 were calculated to be 2.73 eV by the absorption and fluorescence spectra (Fig. S3). This value was found to be lower than that for Spiro-OMeTAD because the introduction of the cyano group induces a decrease in Eg owing to the extension of the absorption band assigned to the π–π* transition, as observed for SF48. 16)
Fig. 2. (Color online) (a) UV–vis absorption spectra of Spiro-OMeTAD (blue), SF48 (violet), and SF62 (red) as solutions in CB. (b) Energy levels of the HTMs. The value for MAPbI3 was obtained from literature, 28) and those for Spiro-OMeTAD and SF48 were obtained from our reported result. 16)
Download figure:
Standard image High-resolution imageThe energy levels of SF62, Spiro-OMeTAD, and SF48 are shown in Fig. 2(b). The highest occupied molecular orbital (HOMO) energy levels of the HTMs were obtained by differential pulse voltammetry (DPV) by dissolving them in CH2Cl2 and tetrabutylammonium perchlorate (TBAClO4) (0.1 M). The lowest unoccupied molecular orbital (LUMO) energy levels were calculated from HOMOs and Eg opt. The HOMO energy level of SF62 was estimated to be −4.75 eV. Although this value is slightly higher than those of Spiro-OMeTAD and SF48, the results show that SF62 has a suitable HOMO energy level for hole extraction from perovskites. Although the LUMO energy level of SF62 was lower than that of Spiro-OMeTAD, it was still sufficiently high to block electron transfer from the perovskite layer to the Au electrode, thus preventing undesired charge recombination.
To confirm the pyrolytic property of SF62, thermogravimetry (TG) and differential thermal analysis (DTA) were conducted (Fig. S4). Although no obvious peak due to the melting process (Tm) was detected for SF62, the crystallization process (Tc) of SF62 was confirmed on the basis of the exothermic peaks observed at 249 °C and a decomposition temperature of ∼300 °C, which are higher than those for of Spiro-OMeTAD. Therefore, SF62 exhibits thermal stability comparable to that of SF48; thus, it is applicable as an effective HTM.
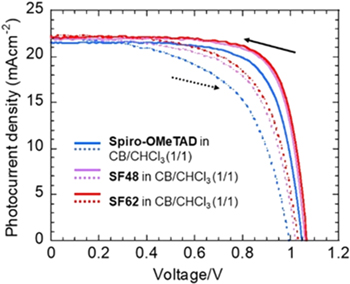
The device structure of fluorine-doped tin oxide (FTO)/c-TiO2/mp-TiO2/perovskite (MAPbI3)/n-octyl ammonium iodide (OAI) 29)/HTM/Au is shown in Fig. S5. First, the cell performances for non-doped Spiro-OMeTAD and SF48 were compared using CB/CHCl3 (1/1) as a solvent. The obtained photovoltaic data of the PSCs are summarized in Table I and SⅡ. The current density–voltage (J–V) curves of the best-performing PSCs with various HTMs are depicted in Fig. 3. The PCEs of the PSCs using SF48 and SF62 were 17.4% and 17.7%, respectively, which were higher than those of the Spiro-OMeTAD-based solar cells under the device fabrication conditions used in this study. For the PSCs prepared using SF48 and SF62, all the parameters showed similar values probably because the HOMO levels of SF48 and SF62 are similar. However, the fill factors (FF) of the PSCs with SF48 and SF62 were higher than those of the ones with Spiro-OMeTAD. SF48 and SF62 may adequately cover the perovskite surface, as discussed later, preventing charge recombination. Therefore, SF62 should be quite effective as a high-performance dopant-free HTM for PSCs, similar to SF48. We used AcOEt, the most common nonhalogenated solvent, as a green solvent for HTM to fabricate PSCs. The obtained data are summarized in Table SⅢ. SF48 is insoluble in AcOEt, whereas Spiro-OMeTAD is slightly soluble (∼5 mg ml−1). By contrast, SF62 is soluble in AcOEt because of its hydrophobic ethyl groups. The PSC fabricated with SF62 exhibited a higher PCE than that fabricated with Spiro-OMeTAD without dopants. All parameters, especially FF, were lower for Spiro-OMeTAD than for SF62. This is probably due to the limited solubility of Spiro-OMeTAD in a solution of HTM, which hampers the formation of a uniform and homogenous HTM layer. Thus, we investigated the J–V characteristics of the fabricated devices as a function of SF62 concentration, because the thickness of the HTL could be controlled by varying the HTM concentration (Fig. S6). The results are summarized in Table SⅣ. Using a thinner HTL afforded higher PCE than using a thicker HTL. However, if the HTL is too thin, reproducibility is reduced. 30) These results are similar to those obtained for SF48. The best PCE was obtained for 7 mg ml−1 SF2 (the thickness of the HTM was ∼20 nm). The J–V curves of the best-performing solar cells are demonstrated in Fig. 4(a), and the J–V characteristics are summarized in Table Ⅱ along with the data for the PSC fabricated using doped Spiro-OMeTAD as a reference. The PCE was 18.6%, higher than those of the doped Spiro-OMeTAD-based PSCs in this study. External quantum efficiency (EQE) spectra are shown in Fig. 4(b). The projected Jsc values of the non-doped Spiro-OMeTAD and SF62-based PSC by integrating the EQE over the AM1.5 G standard spectrum were 21.2 and 22.2 mA cm−2, respectively, consistent with those measured using a solar simulator. The cross-sectional SEM image of the SF62-based device are demonstrated in Fig. 4(c). The optimal HTL thickness for SF62 was ∼20 nm. This value is only ∼10% of the thickness of the commonly used doped Spiro-OMeTAD. When considering the commercialization of PSCs in the future, thinner HTM layers may be preferred in terms of the cost of PSC fabrication. 23)
Table I. Photovoltaic parameters (backward scan) of solar cells for non-doped Spiro-OMeTAD, SF48, and SF62 using CB/CHCl3 (1/1) as the solvent.
| HTM a) | Jsc (mA cm−2) | Voc (V) | FF | PCE (%) |
|---|---|---|---|---|
| Spiro-OMeTAD | 19.7 ± 1.3 b) | 1.00 ± 0.03 | 0.65 ± 0.03 | 12.9 ± 1.7 |
| (21.5) c) | (1.05) | (0.72) | (16.2) | |
| SF48 | 22.0 ± 0.4 | 1.05 ± 0.01 | 0.73 ± 0.01 | 16.9 ± 0.4 |
| (21.9) | (1.06) | (0.75) | (17.4) | |
| SF62 | 20.9 ± 1.0 | 1.04 ± 0.02 | 0.75 ± 0.02 | 16.2 ± 0.9 |
| (22.1) | (1.07) | (0.75) | (17.7) |
a)HTM concentration was 7 mg ml−1 in CB/CHCl3 (1/1). b)These values were obtained from 9 solar cells. (Average ± standard deviation). c)Values obtained from the best-performing solar cell.
Fig. 3. (Color online) J–V curves for the PSCs with non-doped Spiro-OMeTAD (blue), SF48 (violet), and SF62 (red) using CB/CHCl3 (1/1) as the solvent, as measured by forward (dashed) and backward (solid) scans.
Download figure:
Standard image High-resolution imageFig. 4. (Color online) (a) J–V curves for the best-performing PSCs with non-doped SF62 using AcOEt as the solvent, along with doped Spiro-OMeTAD using CB as a reference and (b) EQE spectra of the same PSCs. (c) Cross-sectional SEM image of the SF62-based device.
Download figure:
Standard image High-resolution imageTable II. Photovoltaic parameters a) of PSCs based on non-doped SF62 using AcOEt as the solvent, along with doped Spiro-OMeTAD using CB as a reference.
| Jsc (mA cm−2) | Voc (V) | FF | PCE (%) |
|---|---|---|---|
| Doped Spiro-OMeTAD in CB (reference) b) | |||
| 22.0 ± 0.6 c) | 1.07 ± 0.02 | 0.73 ± 0.03 | 17.2 ± 0.9 |
| (22.3) d) | (1.08) | (0.76) | (18.3) |
| Non-doped SF62 in AcOEt e) | |||
| 22.9 ± 0.3 | 1.04 ± 0.01 | 0.74 ± 0.02 | 17.7 ± 0.6 |
| (23.5) | (1.05) | (0.75) | (18.6) |
a)The scan direction was backward. b) Spiro-OMeTAD concentration (Spiro-OMeTAD) was 72 mM in CB. LiTFSI + TBP was added as a dopant in. c)These values are from 9 solar cells (average ± standard deviation). d)Values obtained from the best-performing solar cells. e)HTM concentration was 7 mg ml−1 in AcOE.
The coverage of the HTM layer on the surface of the perovskite crystal layer without pinholes is crucial for achieving high conversion efficiency of PSCs. 31) Therefore, we examined the surface morphologies of the perovskite layer and the HTL by atomic force microscopy (Fig. S7). The results showed that the perovskite film had a rough surface morphology with a root mean square (RMS) of 18.45 nm. Meanwhile, after coating the HTMs, smoother and more uniform films were formed with smaller RMS values for all cases than those before coating. In particular, the perovskite films coated with SF48 and SF62 showed smaller RMS values than those coated with Spiro-OMeTAD. For SF62, the RMS values were similar when AcOEt and CB/CHCl3 were used as solvents. These results showed that SF62 dissolved in the green solvent AcOEt and both SF62 and SF48 dissolved in CB/CHCl3 can also uniformly cover the surface of the perovskite layer. This suggests that both dope-free SF62 and SF48 are possible to form an effective contact between the perovskite layer and the metal electrode, which in turn reduces charge recombination and enhances charge transfer at the interface. By contrast, Spiro-OMeTAD does not sufficiently cover the surface of the perovskite, which leads to a decrease in cell performance. 23)
Steady-state photoluminescence (PL) and time-resolved photoluminescence (TRPL) were examined to determine the hole extraction capability of the HTMs. Figure S8(a) demonstrates the steady-state PL spectra of perovskite/OAI and those covered with undoped HTMs. The perovskite/OAI shows strong fluorescence, and when it is covered by SF62 using AcOEt as the solvent, the emission signal is more reduced than in other cases. Figure S8(b) shows the TRPL of perovskite/OAI with and without HTMs. The obtained data are summarized in Table SⅤ. The perovskite/OAI covered with SF62 using AcOEt as the solvent exhibited the shortest PL decay lifetime among all tested cases. Meanwhile, perovskite/OAI with Spiro-OMeTAD showed a longer PL decay lifetime than other cases, indicating that Spiro-OMeTAD has poor hole extraction capability. 32) Thus, dopant-free SF62 using AcOEt as the solvent has an excellent hole extraction capability and it affords the corresponding device with high PCE. 33)
Long-term stability of PSCs is considered to be one of the most important issue for future commercialization. 13,34) Thermal stability tests of PSCs with non-doped SF62 were carried out at 85 °C in ambient air (non-encapsulated). As shown in Fig. 5, the thermal stability of the PSC with SF62 was maintained at ∼85% of its maximum PCE after storage for 1000 h (Table SⅥ and Fig. S9). This result indicates that the PSC fabricated with SF62 is as thermally stable as that with SF48, which has already been reported by our group.
Fig. 5. (Color online) Stability performance of the PSC based on non-doped SF62. Non-encapsulated PSCs were kept at 85 °C in ambient air. The obtained values are the average PCEs (backward scans) for 5 solar cells (average ± standard deviation).
Download figure:
Standard image High-resolution imageIn conclusion, herein we developed a new compound, SF62, as an analogue of SF48, and successfully applied it as a dopant-free HTM in PSCs. In particular, the HTL layer of SF62 was prepared using the nonhalogenated green solvent AcOEt, which is known to be one of the most common organic solvents, and only a small amount of SF62 was used for the preparation of the HTM in PSC. Consequently, the PCE of the device based on SF62 was 18.6%, comparable to that of the reference PSC with doped Spiro-OMeTAD (18.3%). Furthermore, the thermal stability of the PSC based on non-doped SF62 at 85 °C in ambient air was found to be superior to that of doped or non-doped Spiro-OMeTAD. From the viewpoint of commercialization of PSCs in the future, SF62 as a dopant-free HTM is expected to have the potential for use in the fabrication of low-cost, environmentally friendly PSCs.
Acknowledgments
The authors thank Ms. W. Tsutsumi, and Mr. H. Nakajima for their assistance with experiments.
Supplementary data (1.1 MB DOCX)